Our Valuable Partners
B&B Dental ITALY
B&B Dental Srl., is a Leading Italian Dental Implant Company located in San Pietro in Casale (Bologna Province) around close Proximity of the manufacturing hub to various global automobile companies of Ferrari, Lamborghini, Maserati and Ducati.
B&B Dental has their owned globally acclaimed state of art manufacturing infrastructure, research and development team bringing in innovative products to the market since 1991. Its Founder Dr.Claudio Banzi a dentist himself have understood the need of innovative design, and surface to the implants design introduced his first design of Dura-vit implants which he availed patents and the story of B&B Dental begun. In same year they availed patents in bone regeneration material called NOVOCOR PLUS

B&B Dental Offers One Stop Solutions for all need in Dental Implantology with the complete spectrum of product offering from Root to Tooth, and Bone regenerative including Sinus Augmentation Tools and Kits and Guided Surgery Solutions product range.
Other innovations in the field of bone regeneration have been introduced through the use of special membranes both in collagen and titanium, effective for the stabilization and protection of the grafted material.
INTERNATIONAL PRESENCE
B&B Dental is present In 60 countries across the world and rapidly expanding across Asia, Mid East and European Sub continent. B&B Dental has distributors across the world who are professionally trained for offering solutions in Implantology. Network of Distributors present across Continents from Europe, Latin America, Mid East & Africa, and Asia Countries.
B&B Dental is a company to watch for rapid growth and innovative products.
BENCHMARK OF QUALITY
B&B. Dental products including bone regenerative range are manufactured in their own infrastructure and are renowned for their highest quality. Each step in the production process is continuously monitored by a sophisticated management system. Quality control protocols known as SPC (Statistical Process Controls), in which the quantity and frequency of the controls (according to the type of product) and the criteria for acceptability are determined.
Sizing checks are carried out using the latest-generation three-dimensional measuring machines, which guarantee accuracy to within ± 0.005 mm (5 microns).
Production quality complies with EN ISO 13485:2003/AC:2009 and Directive 93/42/EEC for Medical Devices. We possess CE Certification and other international standard based on the countries we sell our products.
AROUND 30 YEARS OF INNOVATION
B&B Dental understood the changing need of dental fraternity and constantly introduced with new products offering solutions within dental implantology landscape.
Following are the products which got introduced over the past 30 years:
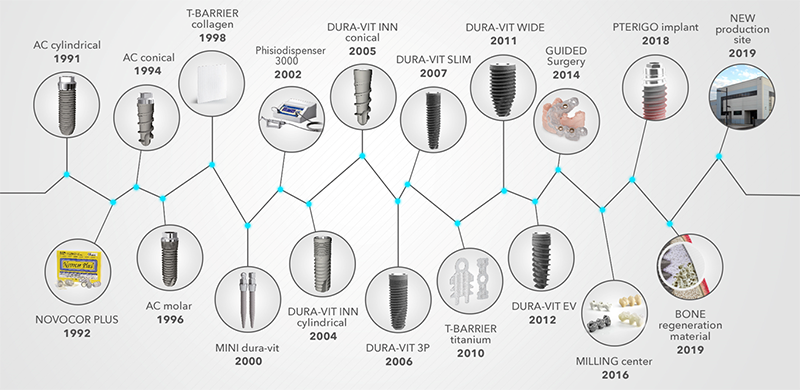
- 1991: AC Cylindrical Implant
- 1992: Novocor Plus Bone regeneration
- 1994: AC Conical implant
- 1996: AC Molar implant
- 1998: T Membrane Collagen
- 2000: Mini Dental implant
- 2002: Phsio-dispencer
- 2004: Dura-Vit Inn Cylindrical
- 2005: Dura-Vit Inn Conical
- 2006: Dura-Vit 3P
- 2007: Dura-Vit Slim
- 2007 : Conexa Revolutionary Connection
- 2010 : T Barrier Titanium
- 2011 : Dura-Vit Wide
- 2012 : Dura-Vit EV (Evolution)
- 2014 : Guided Surgery
- 2016 : Milling Center
- 2018 : Pterigo Implant
- 2019 : Novobone- (Xenografts)
- 2019 : New Production Unit
B&B Dental Launched a new connection in 2007 called CONEXA- with 5* internal tapered connection. This denotes real platform shift with add on benefit of decreased chance in unscrewing of the screws and the prosthetic bacterial colonization at the level of the implant collar. This is how the second generation of systems DURA-VIT INN was born.
In 2009 was put into production the ” third generation ” of Implant EV, SLIM and ø 3, in which was introduced the triple thread which considerably reduces the time of insertion and the passing of the bone at any density . Subsequently, in 2012/2013, were finally conceived the EV and SLIM implants, which provides a double thread groove depth increased for improving the primary stability, especially in spongy bone and thanks to the high self-tapping and self-drilling system, such as to allow an easier insertion and the bone condensation.
Today B&B Dental Offers every solution from CAD CAM Compatible products starting from Titanium base, Scan bodies, Digital Analogues & also integration with the global brands of software for designing and manufacturing prosthesis.
Every dental laboratory who possess the designing software like 3 Shape, Exocad, Dental Wings, egs, Cerec. B&B Dental offers the range of Pre-Milled Abutments to offer customized options for clinicians and dental laboratories.
TRAINING & EDUCATION
Mission to take charge of newer techniques and solutions to dentist more and more interested in “Guided Surgery”.
B & B Dental involve in clinical research in multiple universities in aboard and support clinician with customized educational courses and also with calendar of activities. These are meetings that draw the attention of more and more professionals to the enormous potential of this practice and its several benefits to the patient. Especially in terms of reduced time and success of the intervention.
B. & B. Dental also organizes conferences and courses, with relevant updates, worldwide (countries such as Libya, Yemen, Iraq, Serbia, Russia, Turkey, China, Malaysia, Thailand, Indonesia, Sri Lanka, India, Singapore , Portugal, Italy, Romania and many other countries) achieving success and very positive feedback.
SURFACE CHARACTERISTICS
B. & B. Dental S.r.l. use technological high-level decontamination procedures. The cleaning of equipment surfaces is a complex task. Even using very pure cleaning solvents can still leave surface traces. It can happen that the few existing impurities or the solvent molecules themselves combine with the materials of an implant surface, especially in the case of reactive materials like metals. The ideal cleaning tool should be unable to react chemically with the material of the device and at the same time be very effective for removing contaminants. Argon plasma is the ideal material for this.
Rocky Mountain Tissue Bank USA
The Rocky Mountain Tissue Bank is a Colorado, USA 501c3 non-profit corporation, established in 1980. It is governed by a Board of Directors and is an independent tissue processing facility with no affiliation with any other tissue bank. Shortly Rocky Mountain Tissue Bank is called as RMTB.
They produce irradiated allogenic cancellous bone and marrow for human transplantation, used for bone regeneration in patients with periodontal defects and diseases and provide mainly used in Dental Implants treatments.
A Not for Profit Organisation founded by Dr. William H. Hiatt, who himself a Periodontist was the force behind our creation as a humanitarian organization. His research produced the highest quality and most cost effective transplant material that would stimulate rapid and predictable clinical results.
We will focus on these goals and continue to fund research and education through leading dental and surgical clinicians in the United States and across the world.
Presence: Rocky Mountain is sold across the world and also obtained license to import and sell from the Central Drug Standard Control Organisation (CDSCO) in India. Rocky Mountain is the Only Human Allograft who possess a license to import and sell in India.
Global Registrations/Accreditation





Indications
- Sinus Augmentation
- Sinus Lift
- On-Lay Grafts
- Periodontal Defects
- Segmental Osteotomies
- Tunnel Grafting
- Socket Preservation
- Block Grafting
Dental Allografts
- Cancellous Particulate Vials
- Cortical / Cancellous “Bone Blocks”
- Cortical particulate vials- “bone blocks”
Please click here to Know more about Dental Allografts.
Videos
Dental Allografts: Dr Bernee Dunson Interview at 5th Global AAID Congress
Claronav-NaviDent, headquarters at Toronto- Canada
ClaroNav Inc., A Global Innovative Company for All Navigation Solutions in Medical & Dental Clinical Surgical Procedure for targeting perfection in the area of surgery. Pioneer in the field of Navigation technology in the world with maximum navigation scientific publications and clinical success in Dentistry.
Navigation System tested and proven over decades with 1000s of clinical cases across Medical Science and a few area of have prominent usage are
- Cranio Surgery- Navient Cranial
- ENT Surgery – NaviENT
- Spinal Surgery- NaviSpine
- Dentistry- NaviDent.
NaviDent: Targeting Perfection in various clinical procedures within Dentistry in the area of following:
- Implant Placement, Conventional and Zygomatic Implants
- Sinus Lift Procedures- Crestal and Lateral Window Sinus Elevation
- Vertical Bone Surgeries like Ridge Splits
- Bone Levelling or Bone Grafts harvest sites cutting plan.
- Socket Shield Technique
- Maxillofacial Surgeries- Intra Oral Vertical Ramus Osteotomy etc.,
- Tumor Excisions
- Microsurgical Endodontic Treatment
Navident is now available in India through us to clinicians as exclusive partners for sales, training and support


