DENTAL ALLOGRAFTS
Catalogs
Indications
Our Processing provides you the greatest assurance of allograft quality and safety.
- Sinus Augmentation
- Sinus Lift
- On-Lay Grafts
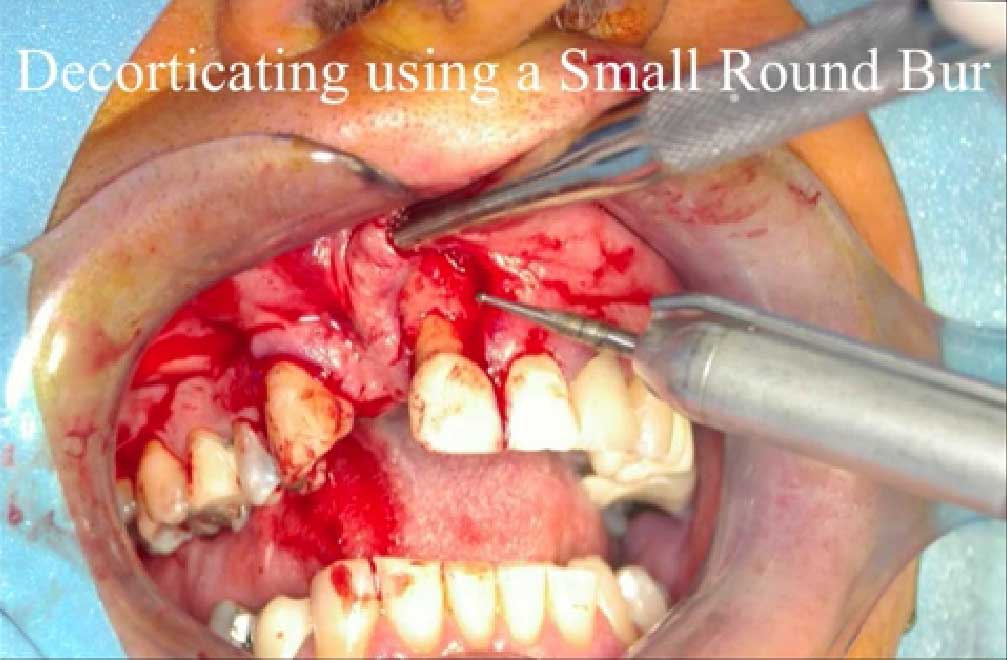
- Periodontal Defects
- Segmental Osteotomies
- Tunnel Grafting
- Socket Preservation
- Block Grafting
Cancellous Particulate Vials
Irradiated Allogenic Cancellous Bone and Marrow Particulate Randomly sized.

- RM-Canpar 0.25mg
- RM-Canpar 0.50mg
- RM-Canpar 1.0 gm
- RM-Canpar 2.0 gm
- RM-Canpar 3.0 gm
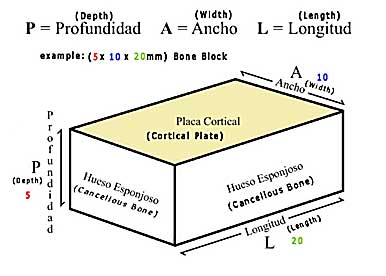
Cortical / Cancellous “Bone Blocks”
Irradiated Allogenic Cortical/Cancellous Bone Blocks

- 5 x 10 x 10 mm RM·BB (Depth x Width x Length)
- 5 x 10 x 20 mm RM·BB
- 5 x 10 x 30 mm RM·BB
- 10 x 10 x10 mm RM·BB (Depth x Width x Length)
- 10 x 10 x 20 mm RM·BB
- 10 x 10 x 30 mm RM·BB
Cortical particulate vials- “bone blocks”
Irradiated Allogenic Crushed Cortical Particulate Randomly sized 2-3 mm.

- RM·CorPar 0.5g
- RM·CorPar 1g
| Periodontal Defects Graft Amount | Extraction Sites Graft Amount | Sinus Grafts | ||
| Central Incisor | 0.25g | Central Incisor | 0.5g | Each Sinus 1 to 3g |
| Lateral Incisor | 0.25g | Lateral Incisor | 0.25g |  |
| Canine | 0.25g | Canine | 0.5g | |
| 1st & 2nd Premolor | 0.25g | 1st & 2nd Premolor | 0.5g | |
| 1st & 2nd Molar | 0.5 g | 1st & 2nd Molar | 1 g | |
| 3rd Molar | 0.5 g | 3rd Molar | 1 g | |
| Rocky Mountain Tissue Bank | ||
| Changing Grams to CC's | ||
| 0.25g | 0.5 cc | www.rmtb.org |
| 0.5g | 0.7 - 1.0 cc | |
| 1 g | 1.2-2.0 cc | |
| 1 to 3 g | 2.5 - 5.0 cc | |
Instructions for Use & Storage with Compliance – Mandatory Requirements
- In the Rocky Mountain Tissue Bank processing, protocol, every effort is made to ensure the safety of our tissue. However, current technologies may not preclude the transmission of infectious agents.
- The use of this tissue is limited to hospitals, physicians, dentists or other qualified medical professionals.
- Allograft has been irradiated with betweeen 25 kGy and 38 kGy of irradiation.
- Do Not Re-sterilize.
- Store at Room Temperature.
- It is the responsibility of the transplant facility and/or the clinician to maintain the allograft in the appropriate storage conditions prior to transplantation.
- The Particulate grafts transport tube should be opened and inner sterilized vial placed in sterile surgical field; or the Bone Block outer pouch is opened and inner pouch is placed in sterile field.
- Once the container seal has been compromised, the tissue shall be either transplanted or discarded.
- No Special Handling is required.
- No additional preparation is necessary for transplantation, such as diluting or reconstitution.
- For Single Patient Use Only.
**Adverse outcomes attributed to the tissue must be reported promptly to rocky mountain tissue bank.
***A patient tracking form accompanies every tissue graft, complete and return it to rocky mountain tissue bank immediately after tissue is transplanted.
****It is the responsibility of the transplant facility and the clinician to maintain recipient records for the purpose of post-transplant tracing.